The AMS scale has been compared with two screening instruments 17: The ADAM scale of Morley et al. 18 and the Screener of Smith et al. 19. The comparison of the AMS of the two screening instruments for androgen deficiency showed sufficiently good compatibility despite conceptual differences of the scales.
The AMS sufficiently predicted the results of the two screening instruments with high specificity but relatively small sensitivity (see table below).
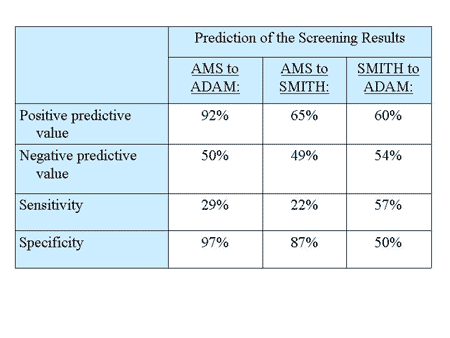 The values for the prediction of Smith’ screener to ADAM results were similar.
The values for the prediction of Smith’ screener to ADAM results were similar.
The AMS scale measures a similar phenomenon as the two established screeners for androgen deficiency. This demonstrates again that the AMS scale has a good criterion-oriented validity.
Can the AMS scale be used as a screening instrument for Androgen Deficiency? Results from the collaboration with Prof. Kratzik of Vienna suggest that this is the case if age and BMI are also considered in addition to the AMS results. 17
17. Heinemann LAJ, Saad F, Heinemann K, DoMinh Thai. Can results of the AMS scale predict those of screening scales for androgen deficiency? Aging Male 2004; Submitted for publication.
18. Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, Perry III HM. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239-42.
19. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in aging men. Clinical Endocrinology 2000; 53:703-11.
 updated
updated