Can the AMS be used to screen populations for androgen deficiency?
The AMS scale was not developed or standardized as a screening instrument5, 25, 26, 29. Moreover, the association between AMS score and testosterone level is controversial with some studies reporting no association30, 31 and others finding a close association 21, 22, 23, 24, 32.
Kratzik et al. 32 have explored the question of whether AMS scores, in combination with age and BMI, could be used as a screening scale and developed a screening tool for low total testosterone level (<4 ng/ml) 37. Kratzik’s aim was to develop a simple instrument for pre-selection of subjects that need further medical diagnostic investigation using a patient-reported self-assessment questionnaire.
A graphical representation of the screening procedure can be seen in the figure below.
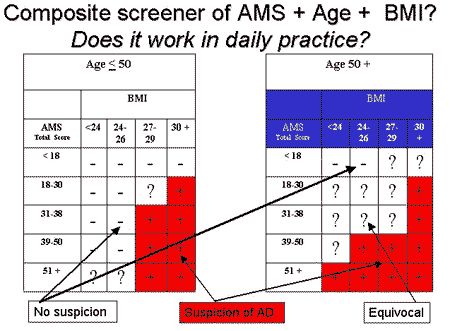
At a screening level we defined three levels of suspicion for androgen deficiency (AD): Positive screening result or high suspicion (+) means contact your physician, equivocal result (?) could lead to the recommendation – wait and contact your physician if complaints persist or increase, and the negative (-) screening result (= no suspicion, AD unlikely) means – no further activities needed. These categories were associated with a different percentage of AD based on TT in blood tests, i.e., 18.7%, 40.7%, and 58.8% in the three screening categories “-“, “?”, and “+”. Smith at al 19 proposed an analogous categorization of the likelihood of androgen deficiency some years ago for their screener for androgen deficiency, the results of which we found to be related even to the AMS scale alone 17.
The quality of a screening test is usually described with sensitivity and specificity. The sensitivity (correct prediction of positive subjects (=low TT value) was 48.3 and the specificity (correct prediction of negative subjects (unsuspicious, high TT value) was 73.7 in the Androx sample where the test was developed. The values in the independent “validation sample” of patients were 69.9% and 64.4% for sensitivity and specificity, respectively.
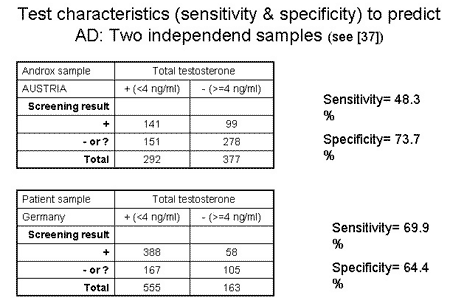
In both samples neither sensitivity nor specificity was very high, i.e. ranging between 50% and 75%. Quite a number of subjects were left in an equivocal situation, for whom further diagnostic workup would be necessary although they were not “positive” according to the proposed screening tool. Nevertheless, the characteristics of new composite screener (AMS+Age+BMI) to detect low TT, seem to be more promising than the AMS values alone, which were reported to be associated with the testosterone level in some 21, 22, 23, 24, 32 but not all studies 30, 31.
The described test characteristics of the proposed composite “AMS- screener” will be acceptable for mass screening and pre-selection of subjects for further diagnostic workup. There might be also an application of this tool for educational purposes or self-assessment through social media. Further research and experience from the practice are needed.
5. Heinemann LAJ, Saad F, Heinemann K, DoMinh Thai. Can results of the AMS scale predict those of screening scales for androgen deficiency? Aging Male 2004; Submitted for publication.
17. Heinemann LAJ, Saad F, Heinemann K, DoMinh Thai. Can results of the AMS scale predict those of screening scales for androgen deficiency? Aging Male 2004; Submitted for publication.
19. Smith KW, Feldman HA, McKinlay JB. Construction and field validation of a self-administered screener for testosterone deficiency (hypogonadism) in aging men. Clinical Endocrinology 2000; 53:703-11.
21. Jankowska EJ, Szklarska A, Lopuszanska M, Medras M (abstract). Male aging and educational level are strong determinants of the intensity of andropausal symptoms in men in Poland. The Aging Male 2004; 7:17.
22. Jankowska EJ, Lopuszanska M, Szklarska A, Medras M (abstract). Hormonal determinants of andropausal symptoms in polish men. The Aging Male 2004; 7:21.
23. Soh J, Ishida Y, Naito Y, Ochiai A, Naya Y, Mizutani Y, Fujito A, Kawauchi A, Fujiwara T, Tschida H, Fukui K, Miki T(abstract). Correlations of AMS score, depression score and hormonal levels with the manifestations of partial androgen decline in the aging male (PADAM).
24. Itoh N, Hisasue S, Kato R, Tanaka T, Takahashi A, Masumori N, Tsukamoto T (abstract). Comparison of Morley’s ADAM questionnaire and Heinemann’s Aging Male Symptoms (AMS) rating scale to screen andropause symptoms in Japanese males.
25. Heinemann LAJ, Saad F, Pöllänen P. Measurement of Quality of Life Specific for Aging Males. In: Schneider HPG (Ed.) Hormone Replacement Therapy and Quality of Life. Parthenon Publishing Group. London, New York, Washington. 2002: 63-83.
26. Heinemann LAJ, Saad F. The aging male’s symptoms scale. In: Schneider HPG (Ed.) Menopause. The state of the art – in research and management. The Parthenon Publishing Group. Boca Raton, London, New York, Washington. 2003: 319-324.
30. T’Sjoen G, Feyen E, Kuyper P, Comhaire F, Kaufman JM. Self –referred patients in an aging male clinic – much more than androgen deficiency alone. Aging Male 2003;6:157-165.
31. Dunbar N, Gruman C, Reisine S, Kenny A. Comparison of two health status measures and their associations with testosterone levels in older men. Aging Male 2001;4:1-7.
32. Kratzik CW, Reiter WJ, Riedl AM, Lunglmayr G, Brandstätter N, Rücklinger E, Metka M, Huber J. Hormone profiles, Body Maß Index and Aging Male Symptoms – results of the Androx Vienna Municipality study. Aging Male 2004;7:188-96.
37. Kratzik C, Heinemann LAJ, Saad F, DoMinh T, Rücklinger E. Composite screener for androgen deficiency related to the Aging Males’ Symptoms scale. Aging Male 2005;8 (3/4):157-161.
 updated
updated