Detection of treatment effect
A summary of the results of the first clinical study where the AMS scale was applied as an outcome measure for testosterone treatment in patients with androgen deficiency is provided below20. Overall, the AMS was able to detect changes in outcome measurements.
On average, the AMS recorded an absolute improvement of symptoms during treatment of 15 points. This was equivalent to an average of 32% of the baseline score.
The relative improvement increased with the degree of severity of symptoms at baseline; 11% relative improvement, 24%, 31%, and 39% improvement in males with little, mild, moderate, and severe complaints at baseline, respectively. Overall, a positive treatment effect was detectable with the AMS scale in men with moderate or even mild symptoms.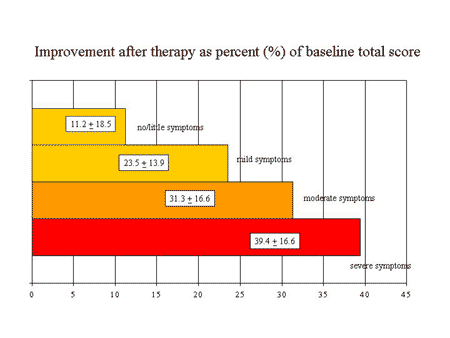 The next figure shows the capacity of the AMS scale to detect therapeutic efficiency from another angle: the comparison with the norm values of the population 5. The level of complaints in elderly patients before therapy is shifted toward a higher degree of severity (higher AMS total score).
The next figure shows the capacity of the AMS scale to detect therapeutic efficiency from another angle: the comparison with the norm values of the population 5. The level of complaints in elderly patients before therapy is shifted toward a higher degree of severity (higher AMS total score).
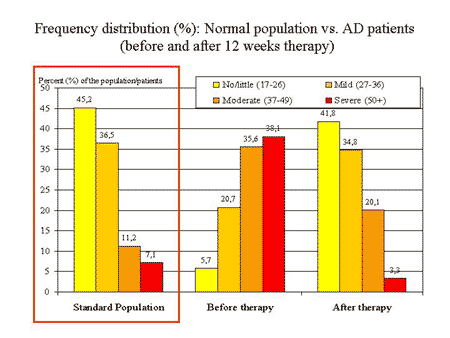 After 12 weeks of testosterone treatment, the frequency distribution of patients with a certain severity of complaints became similar to the distribution in the general population of aging males. This is re-assuring and indicates that comparisons with norm values could be helpful for interpreting results of intervention studies.
After 12 weeks of testosterone treatment, the frequency distribution of patients with a certain severity of complaints became similar to the distribution in the general population of aging males. This is re-assuring and indicates that comparisons with norm values could be helpful for interpreting results of intervention studies.
Prediction of expert assessment
The AMS was also able to predict the independent judgment of the urologists regarding the therapeutic effect. Treating urologists assessed the effectiveness of the hormone treatment in the afore-mentioned intervention study – without prior knowledge of AMS results.
Urologist’s opinions regarding treatment efficiency were divided into two categories: effective and not effective. AMS results were similarly grouped and their correlation assessed. The positive predictive value was 89%, the negative predictive value 59%, sensitivity (correct prediction of a positive assessment by the physician) 96% and specificity (correct prediction of a negative assessment by the physician) 30% 20.
In other words, the change of the AMS score fits well with a positive judgment of the physician concerning therapy efficiency, however, does not predict a negative therapy assessment of the physician well. This means, using the total AMS score as a criterion, it would result in a high positive predictive value but a less satisfactory negative predictive value, very high sensitivity but low specificity.
A subsequent publication from 2006 confirmed this finding. The conclusion of the authors was that the AMS scale showed a convincing ability to measure treatment effects on quality of life across the full range of severity of complaints. Effect modification by other variables at baseline was not observed. In addition, results of the scale could predict the subjective clinical expert opinion on the treatment efficiency.
5. Heinemann LAJ, Saad F, Heinemann K, DoMinh Thai. Can results of the AMS scale predict those of screening scales for androgen deficiency? Aging Male 2004; Submitted for publication.
20. Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, Perry III HM. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239-42.
 updated
updated