The AMS scale is methodologically robust and effective measures and compares health-related quality of life of aging males, over time and before/after treatment. Published data suggest a high reliability and good validity 16.
Reliability
The table below shows the internal consistency measured with Cronbach’s Alpha. The consistency coefficients varied between 0.7 and 0.9 across countries for the total score, as well as the three subscales.
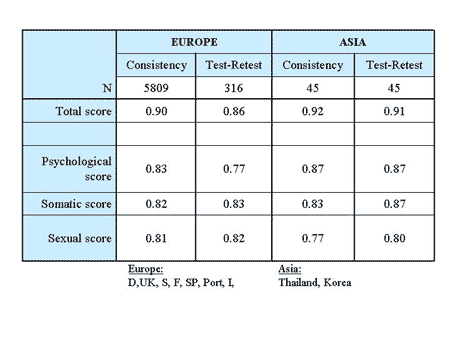
This is indicative of an acceptable consistency of the AMS scale. Moreover, it does not suggest that the scale works differently in different countries in Europe and Asia 16.
In addition, the table shows test-retest correlation coefficients (Pearson’s correlation) which are similarly positive as seen regarding internal consistency. The test-retest coefficients of the total score range between 0.8 and 0.9 across European and Asian countries. However, most of the assessments were based on very small numbers and should be seen as preliminary results only.
In sum, the AMS has passed all reliability tests with good results.
Validity
Validity estimates if a QoL scale really measures what it intends to measure. However, whereas estimates for reliability can be determined with very few indicators, validity is almost always a continuous process (construct validation) based on the accumulation of evidence over time. Several measures can be used to test validity including internal structure, criterion-oriented validity, discriminative or prognostic validity.
Is the internal structure of the AMS consistent across countries and time?
Several analyses have been performed since the first factorial analysis in 1996. The AMS scale has maintained a similar structure over time and different populations since it was first published16, i.e. the scale measures constantly the same phenomenon.
This also holds true for males with androgenic dysfunctions before treatment, i.e. the internal structure of the scale seems to be identical with the “normal population”.
Criterion-oriented validity
The comparison with other scales of similar purpose is an important methodological criterion.
Health-related quality of life like AMS should be validated against quality of life measured with generic QoL scales (e.g., SF36), or against specific instruments to measure symptoms in aging males (e.g. Finnish Turku scales) – or with scales to screen for androgen deficiency (e.g., ADAM, Smith’s scale – see “correlation with ADAM screening scales”). Results of this comparison were published in 2006 by Daig et al.16
Comparison with Finnish Aging Male scales and SF36
Regarding the validity of the AMS scale concerning other aging male scales, a Finnish research group observed a strong and statistically significant correlation with their Turku 14-items scale for aging males (0.8), and similar promising results when comparing with their own “3-Item-Scale”.
The two scales can be regarded, as measuring the same phenomena and this speaks in favor of good test characteristics of the AMS scale 25.
The AMS scale and the generic QoL instrument SF36 were applied at the same time in 116 males aged 40 to 70. The AMS total score and the two sub-scales of SF36 were statistically significantly correlated (-0.49).
The correlation of the somatic sum-score of AMS and of SF36 was sufficiently high (-0.54) as well as the psychological sub-scales of both instruments (-0.65). The correlation is inverse due to the fact that the sum-scores of the AMS increase with symptoms/complaints whereas the sum-scores of SF36 increase with increasing well-being/happiness. But there is no comparator in the SF36 for the sexual sub-scale of the AMS to compare with 25.
Japanese authors investigated the association between AMS results, depression scores, and hormonal levels and found clinically relevant correlations. The AMS scale was suggested for screening purposes in Japanese aging males 23, 24.
16. Daig I, Heinemann LAJ, Kim S, Leungwattanakij S, Badia X, Myon E, Moore C, Saad F, Potthoff P, Do Minh T. The Aging Males’ Symptoms (AMS) scale: Review of its methodological characteristics. Health and Quality of Life Outcomes 2003; 1:77 (December 2003). http://www.hqlo.com/content/1/1/77.
23. Soh J, Ishida Y, Naito Y, Ochiai A, Naya Y, Mizutani Y, Fujito A, Kawauchi A, Fujiwara T, Tschida H, Fukui K, Miki T(abstract). Correlations of AMS score, depression score and hormonal levels with the manifestations of partial androgen decline in the aging male (PADAM).
24. Itoh N, Hisasue S, Kato R, Tanaka T, Takahashi A, Masumori N, Tsukamoto T (abstract). Comparison of Morley’s ADAM questionnaire and Heinemann’s Aging Male Symptoms (AMS) rating scale to screen andropause symptoms in Japanese males.
25. Heinemann LAJ, Saad F, Pöllänen P. Measurement of Quality of Life Specific for Aging Males. In: Schneider HPG (Ed.) Hormone Replacement Therapy and Quality of Life. Parthenon Publishing Group. London, New York, Washington. 2002: 63-83.
 updated
updated