How is the SHE scale scored?
Scoring is straightforward; the score increases point by point with increasing severity/intensity of subjectively perceived complaints in each of the 15 specific items. If items were not present at all “No” (score =1) was coded, if “Yes” was marked, one of four intensity grades have to be chosen (scores 1…5). If a question does not apply to the personal situation, the code “0” (not applicable) can be used.
The score marked in the questionnaire is identical with the scoring points that are added up to the total (or 5 domain) scores.
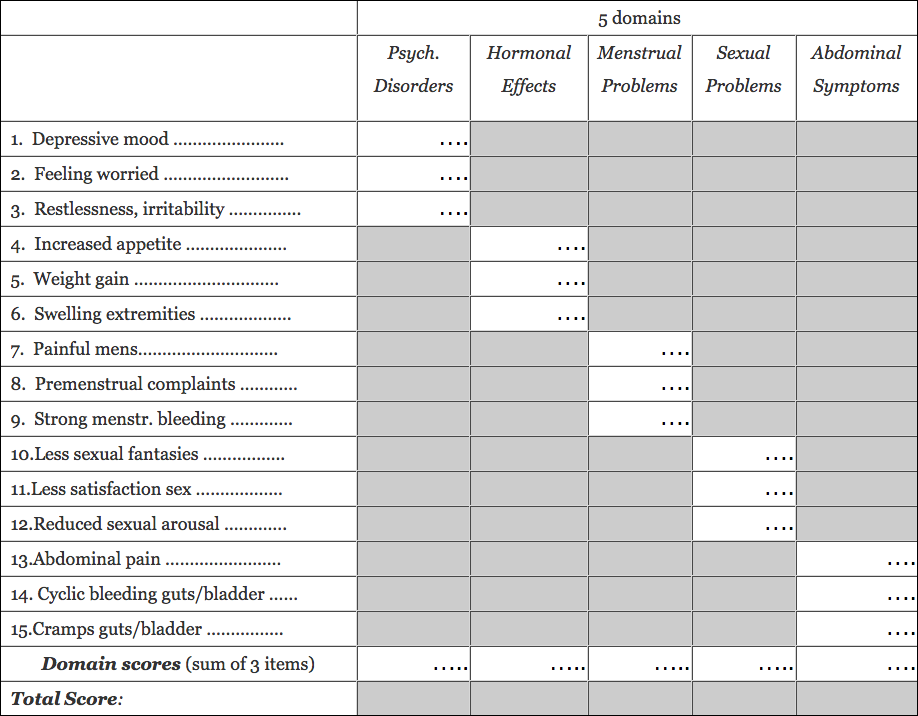
See the evaluation_scheme below. The composite scores for each of the domains (sub-scales) is based on adding up the scores of the items of the respective domains. The composite score (total score) is the sum of the domain scores. This evaluation of the SHE can be done by hand or computerized
The “Short-term Hormonal Effects” (SHE) scale
Once the SHE questionnaire is completed by the women, the following form can be used for an evaluation by hand (or computerized). The scoring scheme of the SHE scale is simple: The questionnaire has for each of the 15 items an option to check one of 5 boxes (coding points 1…5). If a question does not apply, the code “0” (not applicable) might be used.
Write the coding points of each of the items into the form below. The composite scores for each of the five domains are based on adding up the scores of the items of the respective domains. The total composite score (total score) is the sum of the five domain scores. The five domains, i.e. psychological disorders, hormonal effects, menstrual problems, sexual problems, and abdominal complaints, and their corresponding question numbers are detailed in the form.
This form explains how the total sum-score and the sum-scores of the subscales are determined: Add up the points from each of the three items belonging to one of the subscales (indicated by a blank field with dots) to get the sum-score for the respective domains.The “total score” is the sum of the five domain-scores.
 updated
updated